Posted
8th June 2021
Products
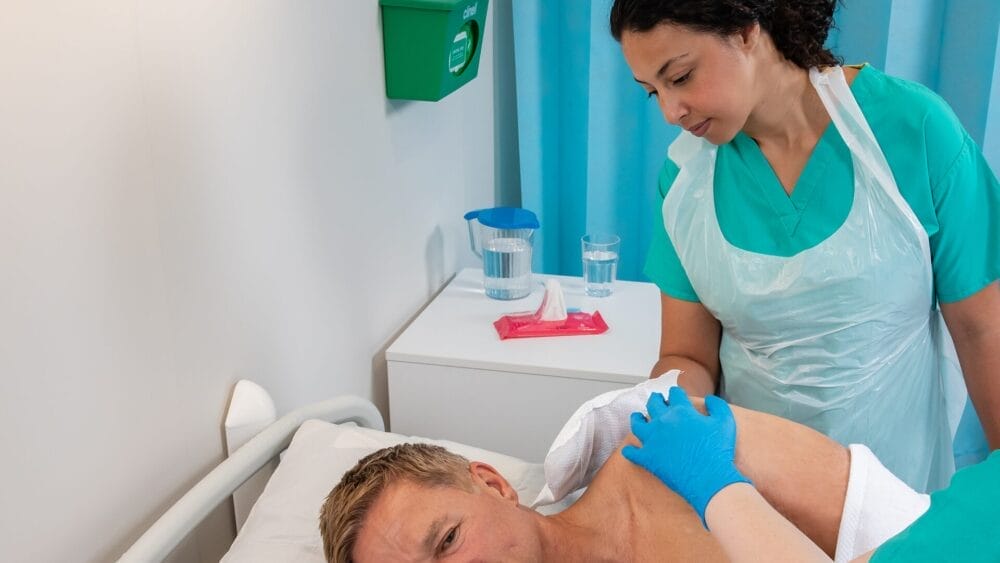
Ref: 2021/005/005/601/530 – Clinell Universal Wipes (CW200)
MHRA published a Field Safety Notice on 20th May 2021 advising of contamination of 5 batches of Clinell Universal Wipe (CW200) with Burkholderia cepacia complex. An update to that Field Safety Notice was published on 4th August 2021 to advise of additional 4 contaminated batches identified during further investigation.
GAMA Healthcare initiated a recall of all 9 identified batches whilst undertaking a comprehensive investigation at the factory. The batches identified were manufactured in June and July 2020 and were distributed between July and December 2020.
GAMA Healthcare have already contacted all customers directly supplied with affected product and issued urgent recall notices to MDSOs in all NHS Hospital Trusts. A copy of the recall notice is available on the MHRA website: https://mhra-gov.filecamp.com/s/Xtu5CzGBSM8ihPbH/d
GAMA Healthcare has also suspended manufacture of the product at the affected site. Manufacture of the product will continue from our other unaffected site and so the supply of CW200 will not be disrupted as a result of this suspension.
For any queries about this recall, please contact GAMA Regulatory Team; regulatory@gamahealthcare.com, or by calling GAMA by phone on +44 (0) 207 993 0030 and selecting the Regulatory option from the menu.
SHARE THIS ARTICLE
Tags
Latest News
Introducing HEXI HUB: A seamless transition in our product line
We’re pleased to announce an update to our product offering…
Innovative solutions for tackling Carbapenemase-producing Enterobacteriaceae (CPE) at King’s College Hospitals
King’s College Hospital NHS Foundation Trust, one of London’s largest…
Gloves Off: reducing unnecessary plastic waste during environmental cleaning and disinfection
In this blog, Dr Phil Norville discusses the momentum-gaining ‘Gloves…
Gloves Off: Navigating SDS sheets and skin safety claims in environmental decontamination products
In this blog, James Clarke (Head of R&D, Science &…