Posted
28th July 2022
Research
Manufacturer claims don’t always reflect real-world use. But ineffective surface disinfection puts patients at risk and contributes to the £2.7 billion spent on healthcare-associated infections by the NHS. We look at the questions to ask when it comes to selecting an effective disinfectant product for healthcare.
Manufacturers make bold claims, based on laboratory testing, that don’t reflect real-world use. When we don’t ask the right questions, we can end up with a product that’s ineffective in the real world, puts patients at risk and costs healthcare organisations extra time & money.
Healthcare-associated infections:
– Cost the NHS £2.7 billion per year
– 28,500 patient deaths in England
– 79,700 healthcare worker absences
Statistics demonstrating the annual cost of healthcare-associated infections to England’s National Healthcare Service. Guest JF, Keating T, Gould D, Wigglesworth N. Modelling the annual NHS costs and outcomes attributable to healthcare-associated infections in England. BMJOpen. 2020;10:e033367.
Surface disinfection is an evidence-based practice. Improving environmental cleaning is one of the most cost-effective ways to improve patient outcomes[1]. But there are some important questions that healthcare organisations need to be empowered to ask disinfectant manufacturers.
Industry leaders including Peter Hoffman and the Royal College of Nursing have contributed to criteria that organisations should use to evaluate surface disinfection solutions:
1. Did you test the liquid off the wipe, or the liquid that goes into the wipe?
Wipe substrate (the material the wipe is made of) can trap the active ingredients of a disinfectant. This process (known as “adsorption”) renders some efficacy data invalid.
When disinfectant manufacturers use the pure liquid formulation for efficacy testing, the product will appear more effective.
However, in the real world, because active ingredients remain trapped in the wipe, the concentration of active disinfectant delivered to the surface becomes much lower. This allows microorganisms to survive.
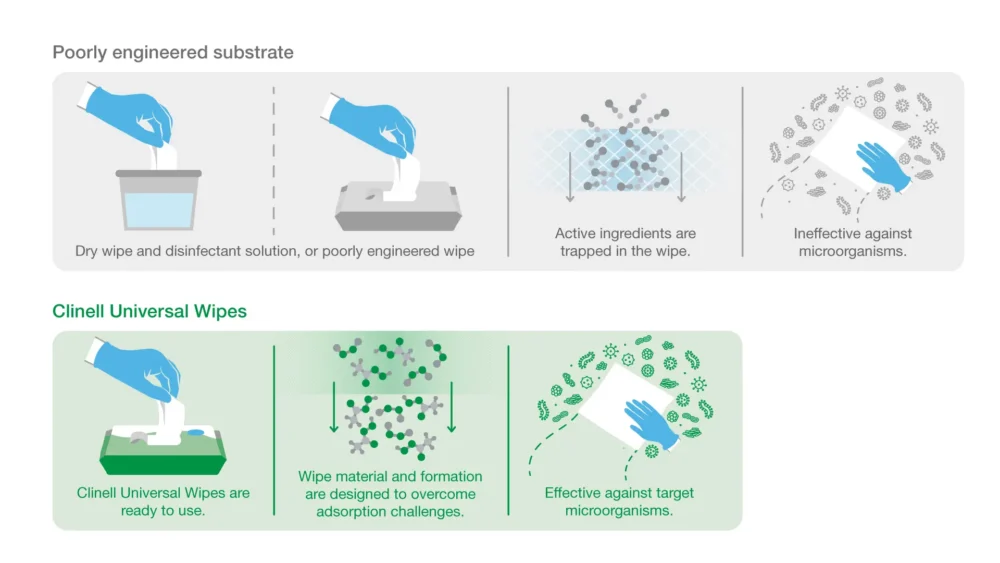
Our approach: all our Clinell Universal Wipe product are tested using liquid extracted from the wipe (eluate) – this means efficacy tests reflect real-world usage.
“Was efficacy testing for this product conducted on liquid extracted from the wipe?”
2. Have you tested against clinically relevant test organisms?
UK and EU regulations specify minimum test organisms required to make biocidal claims. For example, to make the blanket claim “kills 99.999% of bacteria,” for surface disinfectants, efficacy testing is only required for 3 bacterial species (S. aureus, E. hirae and P. aeruginosa).
These minimum test organisms exclude many clinically relevant pathogens and MDROs. Including Candida auris, Acinetobacter baumannii and CPE.
Our approach: Clinell Universal Wipes (and other Clinell disinfectant products) feature extensive test data for clinically relevant pathogens. For a full list of efficacy data, see our Clinell Universal Range page.
“What clinically relevant organisms has this product been tested against?” “Has it been tested against Candida auris? CPE? VRE?”
3. Are the contact times realistic?
Disinfectants don’t work instantly. They require time in contact with a microorganism to be effective. Every organism tested will have its own contact time.
Liquid disinfectants (including pre-impregnated wipes) are only effective when wet. If the liquid on a surface dries before the contact time is reached, pathogens will survive disinfection.
Unfortunately, many standard efficacy tests allow contact times of 5 minutes (even up to 60 minutes). This is unrealistic in real-world practice.
Our approach: GAMA Healthcare conducts efficacy testing within realistic contact times
For a full list of efficacy data, see our Clinell Universal Range page.
“What is the specified contact time for each organism? Is that realistic?”
4. Was it tested in dirty conditions?
Disinfectant manufacturers can manipulate efficacy data by testing in clean conditions.
Organic matter can inactivate disinfectants – this is why chlorine and alcohol-based disinfectants require a pre-cleaning step.
Disinfectants tested in clean conditions will appear to be more effective in shorter contact times. However, this does not reflect real-world usage for 2-in-1 cleaning & disinfectant wipes.
2-in-1 cleaning & disinfectant wipes should always be tested in dirty conditions. These products are expected to remove dirt from surfaces and disinfect them in a single wipe. If they don’t work in dirty conditions, they’re not going to be effective in real-world use.
Our approach: Our efficacy testing has been conducted, in dirty conditions, at university and accredited laboratories, according to standardised test methods outlined by the European Union and the UK government. All results are validated.
“Was this test conducted in clean or dirty conditions?” “Where was this test conducted? Was the lab accredited to conduct this test?”
5. Is it supported by published evidence?
Whilst lab-based efficacy testing provides important insights, effective products should be backed up with real-world, peer-reviewed studies.
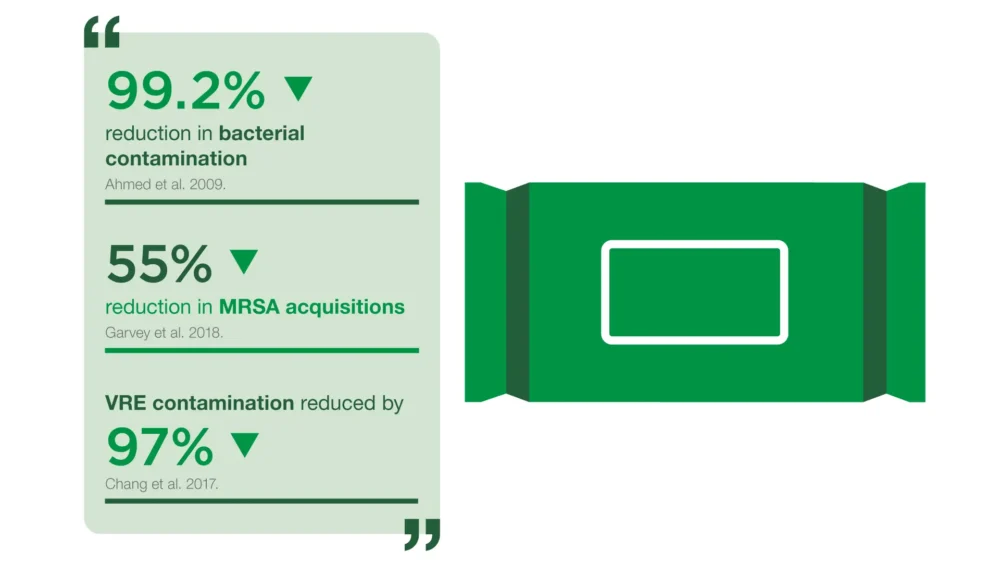
Our approach: Clinell Universal Wipes are proven to MRSA acquisition[2], reduce multi-drug resistant organisms in the environment[3] and save staff time[4].
“What real-world, peer-reviewed studies have examined the effectiveness of this product?”
6. Have you considered material compatibility with surfaces?
Poorly formulated disinfectants can damage surfaces. In certain circumstances, repeated disinfection can cause medical devices to fail prematurely.
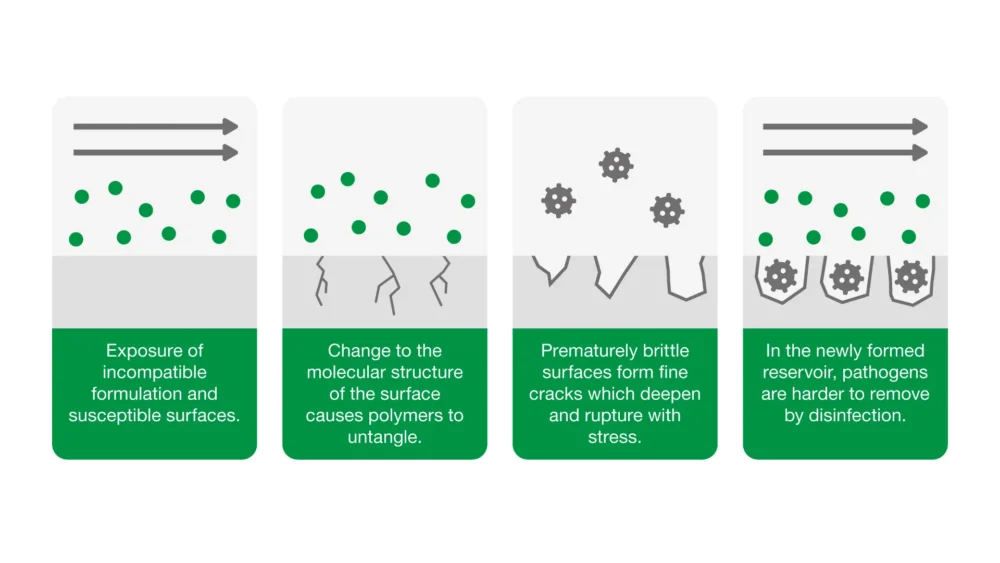
Our approach: Our Material Science team conduct extensive surface compatibility testing between our Clinell products and the most common surface materials found in healthcare – an array of plastics, metals and rubbers.
We work in partnership with medical device manufacturers to conduct compatibility testing. We have approval for over 100 medical devices, including products from BD (Bard), GE Healthcare, Agile medical, Philips, Welch Allyn and many more.
See full list of approved manufacturers.
“Have you conducted surface material compatibility testing? Could you share the results of that data?” “What approvals do you have from medical device manufacturers?”
7. What about manufacturing quality & regulatory compliance?
A single hospital can use thousands of disinfectant wipes daily to protect their patients, visitors and staff. Each wipe needs to be efficacious and free from contamination.
For use on medical devices, disinfectant wipes should be CE-marked and registered as class IIa medical devices.
Our approach: We works with accredited suppliers and conducts frequent quality assurance audits in all manufacturing facilities. Our facilities use advanced filtration techniques to remove bioburden in our disinfectant liquid, from our wipe substrate and packaging.
“Is this product a CE-marked medical device?” “What quality and hygiene controls do you have in place?”
8. How do you approach training, implementation & support?
Good products are only effective when supported by good practice.
When we support staff with training in environmental decontamination, we can reduce the spread of pathogens like MRSA[2] and VRE[5] and save staff time spent cleaning[4].
Implementation and ongoing support have a profound impact on everyday clinical practice.
Our approach: GAMA Healthcare is well-practiced at supporting large-scale product conversions. We offer market-leading ongoing support including: face-to-face training from our Clinical Educators, e-learning, bespoke awareness materials & instructional posters and powerful audit tools – all free of charge to Clinell customers.

This year:
8,409 NHS staff trained
by our IPC Nurse Trainers and Clinical Educators
“What successes have you had implementing product into organisations like this?” “What is your training and support offering?”
Find out more
We’ve put together a handy reference sheet of questions to ask and why.

To find out more about the evidence for Clinell Universal Wipes or Clinell Peracetic Acid Wipes, speak to your local GAMA Healthcare Area Manager or contact us.
[1] White NM, Barnett AG, Hall L, et al. Cost-effectiveness of an environmental cleaning bundle for reducing healthcare-associated infections. Clinical Infectious Diseases. 2020;70(12):2461-2468. doi:10.1093/cid/ciz717
[2] Garvey MI, Wilkinson MAC, Bradley CW, Holden KL, Holden E. Wiping out MRSA: Effect of introducing a universal disinfection wipe in a large UK teaching hospital. Antimicrobial Resistance and Infection Control. 2018;7(1). doi:10.1186/s13756-018-0445-7
[3] Chang F, Lin C, Lee C. A comparison of 1000ppm Chlorine and single-use detergent/disinfectant wipes for decontamination of a clinical environment contaminated with Vancomycin-resistant Enterococci. Journal of Infection Prevention. 2017;18(1S):S14.
[4] Shepherd E, Leitch A, Curran E. A quality improvement project to standardise decontamination procedures in a single NHS board in Scotland. Journal of Infection Prevention. 2020;21(6):241-246. doi:10.1177/1757177420947477
[5] Mitchell BG, Hall L, White N, et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): a multicentre, randomised trial. The Lancet Infectious Diseases. 2019;19(4):410-418. doi:10.1016/S1473-3099(18)30714-X
SHARE THIS ARTICLE
Tags
Latest News
Celebrating 20 Years of GAMA Healthcare: Our Story
This month, GAMA Healthcare celebrates 20 years of helping prevent…
Norovirus: Understanding its transmission and prevention in the UK
Introduction Norovirus is recognised as the leading cause of viral gastroenteritis…
Clean Between to Reduce Healthcare-Associated Infections
Healthcare-associated infections (HAIs) are a significant concern for healthcare facilities…
Mpox: emergence of a new threat
A new threat related to mpox is emerging, in the…